Advanced nanostructured materials for industrial carbon capture
In this application example we show how Raman spectroscopy is being used to investigate nanostructured materials for carbon capture. With climate change and rising CO2 emissions, governments are looking at new ways to reduce atmospheric CO2 concentrations.
Currently, CCUS technologies are at varying levels of maturity. For example, amine based CO2 capture is already deployed at large scale during chemical production.1 Other emerging technologies have the potential for better performance at lower unit costs, but still require further development.
Here we discuss two novel materials with promising performance characteristics for carbon capture: solid-amine nanoporous structures and graphene membrane filters. Raman spectroscopy is vital for characterising and developing these cutting-edge materials. Renishaw's range of spectrometers provide chemical and structural characterisation so that the synthesis of these advanced materials can be successfully optimised.
Solid-amine based carbon capture
Amine scrubbing has been applied since 1930 and allows CO2 collection with high purity (>99%).2 Amine scrubbing is the process of removing hydrogen sulfide (H2S) and carbon dioxide (CO2) from flue gases using aqueous solutions of amines. Typically, CO2 is captured by using monoethanolamine solution (20–30 wt % in water) and then released at high temperatures of 100–120 °C. This process produces a huge amount of degraded solvent as waste. Therefore, the current scrubbing technologies are energy intensive and uneconomical. As a result, there is renewed interest in solid-amine materials for carbon capture.
Carbon-based nanoporous structures are particularly attractive as a solid amine-based CO2 capture system, due to their relatively high thermal stability, chemical resistance and potential for large-scale production at low-cost. A high percentage of porosity can be engineered to readily achieve large amounts of amine impregnation for effective CO2 absorption.
Professor Zheng-Xiao Guo and his co-workers at University College London, UK, have reported a scalable and well-controlled method for obtaining carbon-based nanoporous structures impregnated with amine. They have synthesised highly hierarchical meso- and macro-porous graphene networks, prepared by thermal-shock exfoliation of graphene oxide (GO) at moderate temperatures of 300˚C for a short time of about 5 minutes.3 The degree of oxidation of the GO controls the strength of exfoliation to yield extraordinary porosity: both the specific surface area (SSA, ≈800 m2 g−1) and ultrahigh total pore volume (≥6 cm3 g−1) are significantly higher than for other mesoporous carbons, metal-organic frameworks (MOFs), silicas and zeolites. Raman analysis of the D- and G-bands of GO were performed on an inVia™ confocal Raman microscope to confirm the varying degrees of chemical modification before and after exfoliation (Figure 1).
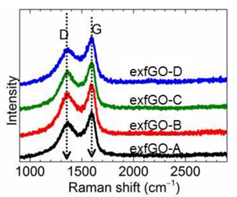
Figure 1: Raman spectra of the exfoliated graphene oxide (exfGO) samples represent a highly disordered state of the samples due to chemical modification. The exfGO samples are labelled A to D in order of increasing oxidation state. Note that the 2D-band of graphene (≈2700 cm−1) is barely detected, indicating a loss of structure (order).
(From: c7ta05789j1.pdf (rsc.org))
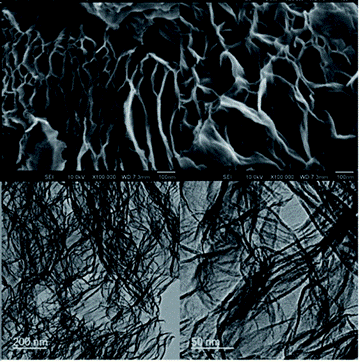
Figure 2: Highly networked surface morphology and porosity of exfGO-D samples determined by electron microscopy. The upper and lower rows show the scanning electron microscope (SEM) and tunnelling electron microscope (TEM) micrographs, respectively.
(From: Design of hyperporous graphene networks and their application in solid-amine based carbon capture systems - Journal of Materials Chemistry A (RSC Publishing) DOI:10.1039/C7TA05789J))
The exfoliated GO samples were impregnated with triethylenetetramine (TETA). When the samples were tested in a simulated flue-gas stream (75C, 100 ml min-1, 15% of CO2 in N2, bubbled through water), a stable working CO2 capture capacity of >25 wt% (7.0 mmol g-1) was achieved. These values are better than any other literature values for any type of porous solid or solid-amine carbon capture system.3
An efficient and cost-effective graphene membrane filter
Membrane filters are attractive as an energy-efficient solution for carbon capture. The challenge of achieving molecular separation by gas-sieving through nanoporous single-layer graphene has been researched for over 10 years4. More recently, Professor Kumar Varoon Agrawal and his team of chemical engineers at Ecole Polytechnique Federale de Lausanne (EPFL) have developed a highly selective graphene filter membrane for carbon capture. This graphene filter surpasses the efficiency of existing commercial carbon capture technologies, at a significantly reduced cost of $30 per ton of carbon dioxide.5
The highly controlled synthesis method devised by Prof Agrawal's group can achieve a high pore density and a narrow pore-size distribution (PSD) across graphene monolayers. This translates to sub-angstrom resolution for molecular differentiation between similar-sized molecules: CO2/N2; CO2/CH4; O2/N2. The resulting graphene membranes show excellent sieving performance with large CO2 and O2 permeance. The membranes are therefore a scalable and an energy-efficient material for carbon capture.
Raman spectroscopy was used to characterise the nanoporous single layer graphene (N-SLG) films, which was synthesised by treatment with O3 in a reactor.6 The high sensitivity and fast mapping capabilities of Renishaw's inVia confocal Raman microscope enabled the structural characterisation of the nanoporous single layer graphene films. Raman analysis revealed ID/ID' ratios below 3, which indicates that most of the defects were edge-like defects of graphite (Figure 3). Fast Raman imaging was used to measure the ID/IG ratio, thus indicating that the porous defects were uniformly generated over a large area.
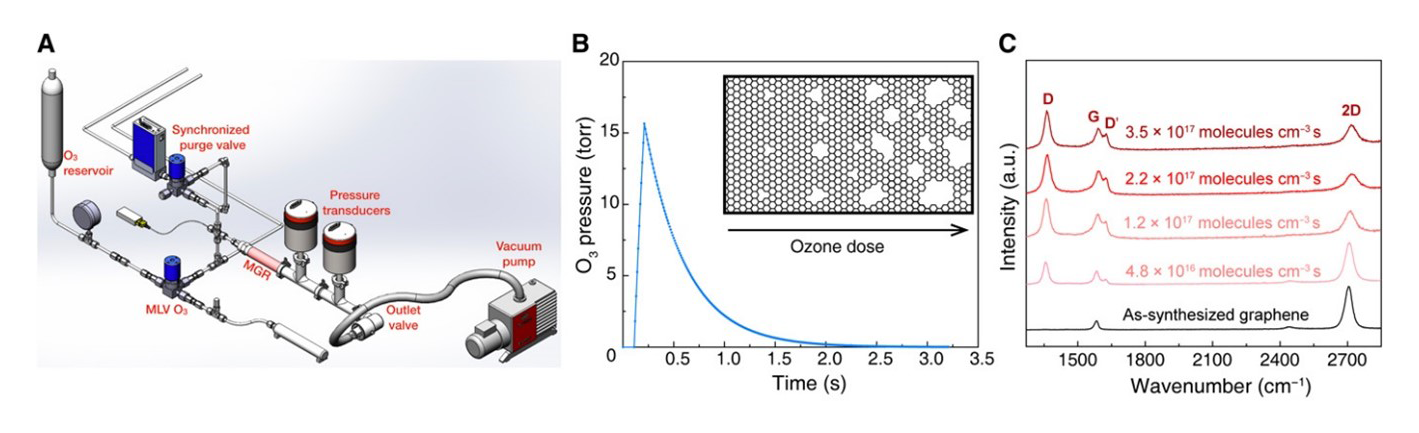
Figure 3: Precise incorporation of a high density of vacancy defects in nanoporous single layer graphene by millisecond gasification with O3. (A) Schematic of the reactor setup. (B) Profile of the O3 pulse in the reactor. (C) Raman spectroscopy analysis showing the evolution of the N-SLG with increasing O3 dose.
New materials can help to decarbonise industrial processes
The urgency of decarbonising industrial processes and fossil fuel power generation is driving the development of new functional materials. Raman spectroscopy can help researchers to optimise the chemistry and structure of nanostructured materials. Various promising technologies are being developed for carbon capture applications, and we are excited to see how Raman analysis is used to meet this challenge.
References:
(1) CCUS technology innovation CCUS in Clean Energy Transitions Analysis - IEA
(2) Review on CO2 Capture Using Amine-Functionalized Materials | ACS Omega
(4) Selective molecular sieving through porous graphene | Nature Nanotechnology
(5) Graphene filter makes carbon capture more efficient and cheaper (phys.org)